What is covered in Cleaning Validation training?
We cover planning, sampling techniques, analytical methods, acceptance criteria, re‑validation triggers and documentation best practices.
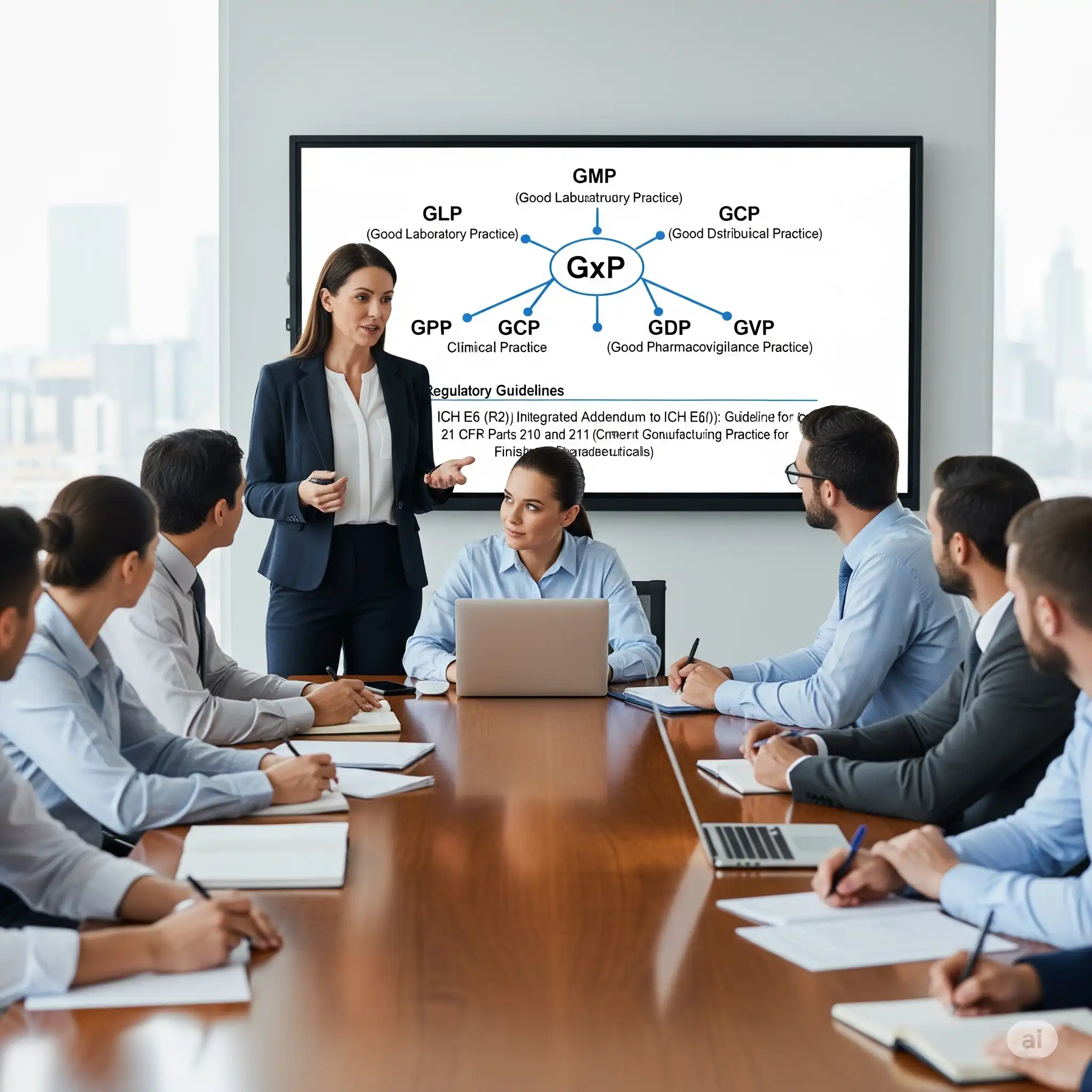
What topics are included in GxP training?
Our GxP module reviews regulatory requirements under GLP, GMP, GCP and GDP, with practical advice for compliance in lab, manufacturing and distribution.
Why is Revised Schedule M training important?
It helps teams understand new Indian GMP standards on quality systems, risk management, HVAC, data integrity and documentation.
What do WHO TRS guidelines cover?
WHO TRS Annexures provide detailed expectations on GMP, quality risk management, sampling, validation, and trouble‑shooting guidance for pharma.
How do you train on pharma data integrity?
Training includes ALCOA+ principles, data lifecycle, audit trails, documentation controls, self‑inspection and CAPA.
What is a Quality Management System (QMS)?
QMS is a structured system for all quality-related activities: document mgmt, audit, CAPA, training, change control and continuous improvement.
What topics are covered in your cleaning validation training?
Our cleaning validation training covers principles, worst-case product selection, residue limits (MACO, PDE), swab and rinse sampling, cleaning method validation, protocol development, and regulatory expectations from FDA, EMA, WHO, and PIC/S.
How do your GxP trainings benefit pharmaceutical teams?
GxP trainings (GMP, GLP, GCP, GDP, GVP) equip teams with knowledge of global standards, ensuring compliance, improving operational efficiency, and preparing staff for regulatory inspections.
What is the duration of your training programs?
Training sessions typically last 1–3 days per topic, with customizable modular delivery to suit client schedules and specific needs.
How do you ensure training aligns with regulatory requirements?
Our programs are designed to comply with FDA 21 CFR Parts 210/211, EU GMP Annexes, WHO TRS, Schedule M, and ICH Q9/Q10/Q14, with training certificates and documentation suitable for audits.
Who should attend your quality management systems (QMS) training?
QMS training is ideal for QA, QC, production, warehouse, R&D, and regulatory affairs teams in pharmaceuticals, biotech, and CROs seeking to implement or optimize ICH Q10-compliant systems.
Can your training programs be customized for specific roles?
Yes, we offer tailored modules for QA, QC, production, R&D, and regulatory affairs, with options for on-site, virtual, or hybrid delivery, including workshops and role-specific simulations.